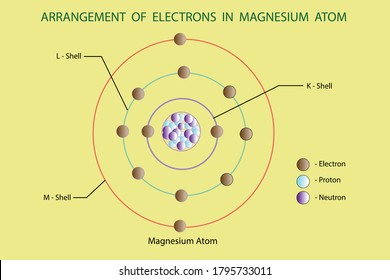
There are three shells in magnesium atom and there are two electrons in the first shell, eight in the second shell and two in the third shell. There are total 12 electrons in magnesium atom and 2 electrons are present in the outermost shell of magnesium. For more details: Magnesium atom


Mg Atom Before The Reaction
2021: Electron Configuration of Magnesium (Mg) Complete, Abbreviated, Uses. Electrons have a specific form of distribution (or configuration) in every atom, even Magnesium. Some are hard to memorise (or predict), so what is the electron configuration of an atom of Mg? In the case of Magnesium the abbreviated electron configuration is Ne 3s2. Magnesium is in group 2 of the periodic table. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Chlorine is in group 7 of the periodic table. A chlorine atom will gain 1 electron to form a stable 1 - ion. 1 mole is equal to 6.0221415E+23 atom. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between moles and atoms. Type in your own numbers in the form to convert the units! ›› Quick conversion chart of moles to atom. 1 moles to atom = 6.0221415E+23 atom. 2 moles to atom = 1.2044283E+24 atom. Magnesium (12 Mg) naturally occurs in three stable isotopes, 24 Mg, 25 Mg, and 26 Mg. There are 18 radioisotopes that have been discovered, ranging from 19 Mg to 40 Mg. The longest-lived radioisotope is 28 Mg with a half-life of 20.915 hours. Although magnesium (Mg) is an essential element for plant growth, its use in a fertilizer program receives only minor emphasis in Minnesota. For most of the state, this lack of emphasis is justifiable because when management properly, most soils in Minnesota contain sufficient Mg to meet crop needs.
Mg Atomic Weight
Didn't find the answer you were looking for?

